

What Early Land Plants Can Tell Us About Earth's Family Tree
The plant pioneers that moved from water to land had to change their way of life in order to adapt and survive. A team of biologists aims to find out which came first and how they evolved.
Half a billion years ago, the land on Earth was barren rock and ash. Only the water-the oceans, shallow seas, and lakes, teeming with simple aquatic plants, invertebrates, and primitive jawless fishes-held the secret of life.
Then somewhere, somehow, a brave new kind of green algae got a grip on the rocky shore. That momentous step, which may have happened in many places in various ways, made possible the world we know. We owe our existence to the land plants that evolved subsequently-to the oxygen they pump out, the nutrients they make and store, the soil they build, the animals they feed and shelter.
How did plants get that initial toehold on land, survive, and go on to diversify into the lush life we take for granted? A group of scientists dubbed "Deep Green" is dedicated to finding out.
"Deep," in this case, means deep in time. These biologists are fascinated with the "green" family because of its age and its central place in life on earth.
How did multicellular aquatic plants evolve? Which plants first colonized land? How are the early plant lineages related to each other? What genetic, cellular, and structural changes did they undergo?
With funding from the National Science Foundation's "Tree of Life" initiative, nine "Deep Green" scientists at SIUC and other institutions are tackling these questions to reconstruct in detail the family tree of the plant kingdom. The five-year, $3 million "genealogical" study is focusing on plant groups with an ancient heritage, such as algae, mosses, and ferns, where evolutionary relationships are fuzzy at best and where the most dramatic biological changes had to occur.
"If we can understand the relationship of living organisms-put together a 'big picture' of how life evolved-we can answer some important biological questions, which could influence everything from improving human health to managing the environment effectively," says SIUC plant biologist Karen Renzaglia, a member of the team.
Renzaglia, who will receive $375,000 from the NSF for her part of the research, studies cell-level changes in early land plants. "The constraints of living on land are pretty incredible," she says. "You have to figure out how to hold your body up against gravity, how to keep from drying out, how to move water and chemicals around in the body-and how to do sex out of the water!"
The earliest land plants to begin solving those problems are still the smallest: mosses, liverworts, and hornworts. Collectively called bryophytes, they're found on every continent but Antarctica. They hug the ground, absorbing water and nutrients directly through their cells. Skinny threads called rhizoids, precursors of root systems, anchor them to the soil.
Only after plants evolved true root systems and veins to ferry around water and nutrients could they grow very big. Giant tree ferns lifted their fronds up to the sun; rush-like horsetails and club mosses developed tall spore-bearing cones. These new types of plants, collectively called pteridophytes (Greek for "fern plants"), changed Earth's landscape dramatically.
Bryophytes and pteridophytes may have conquered the land, but they still rely on a film of water for reproduction. In mosses and their relatives, the plant produces sex organs containing sperm and egg cells. When the sperm are released, they "swim" to eggs on the same plant or nearby plants. Each fertilized egg sprouts a thin, shortlived stalk that bears spores along its sides or in a capsule at its tip. The spores fall to earth, where they germinate and produce new plants.
In ferns and their relatives, that sequence is reversed: The mature plant makes the spores. Turn over a fern frond and you'll see neat dots of spores bracketing the veins. When those spores fall to the soil, they each sprout a tiny plant-a mere speck on the forest floor or even underground-that exists to make egg and sperm cells. The sperm wiggle their way through water drops to fertilize nearby eggs, which then sprout new ferns.
Not until the evolution of seed-bearing plants, some millions of years later, did plant species free themselves from the need for water to get sperm and egg together. Seed plants-the trees, flowers, and grasses so familiar to us-package sperm in pollen, an ingenious solution that also enables them to reproduce at a distance.
Beyond these broad outlines, little is known for certain about early plant evolution.
"Major portions of this evolutionary tree are ambiguous," says Renzaglia. "There are so many gaps in our knowledge about the life cycles, structure, and development of early land plants. And almost nothing is known about the cellular details. These plants have evolved some fundamentally different mechanisms for [cell division and reproduction] that will give us information about evolutionary relationships."
Renzaglia studies the morphology (form and structure) and cell biology of plants. She has gained a worldwide reputation for her studies of sperm cells in bryophytes and pteridophytes. "Nobody knew the diversity of sperm in plants before we started doing this work," she says. Similarities and differences in these cells across species tell her a lot about when different groups of plants evolved and how they are related to each other.
Renzaglia suspects that the lowly hornwort, which coats itself with a water-holding layer of slime, was the earliest land plant that still exists today. In a separate project also funded by the NSF, she and biologist Joel Duff at the University of Akron are piecing together the family tree of the world's 150 or so kinds of hornworts.
So little is known about hornworts, she says, that "it's hard to know what defines a species or genus." Her morphological work, plus Duff's data on gene sequences and proteins that occur in these plants, are overturning current classification schemes.
"We never predicted the relationships we're coming up with," she says, "but they make sense in terms of which is the oldest hornwort and how the group developed."
Why sort out the hornworts? Why try to figure out the evolutionary pathways of the earliest land plants at all?
On an economic level, this information will help efforts in, for example, drug prospecting. If you identify one hornwort that produces a pharmaceutically useful compound, you'll want to zero in on that species' closest relatives so that you can analyze them for similar compounds.
On the environmental level, efforts to conserve biodiversity and save species will rely on knowledge about genetic relationships. Because we can't save everything, we should try to preserve the greatest genetic diversity, Renzaglia says. Species that are very distinctive genetically, with no close relatives, should take higher priority than species with abundant close relatives.
On a broader level, determining the history of early land plants will tell biologists more about how life forms function and have developed (see sidebar).
The "Deep Green" team has chosen for in-depth analysis some 50 species of plants that they believe represent major lineages among green algae, bryophytes, and pteridophytes. The team's leader, Charles O'Kelly of the Bigelow Laboratory for Ocean Sciences (Maine), is studying the morphology of the algae species. Renzaglia and her students are doing the same for the others.
To understand all the developmental stages of these plants, they're scrutinizing spores and spore capsules, egg and sperm cells, embryos, and mature plants. They're documenting dozens of characteristics, photographing specimens, comparing and compiling details previously reported about the species in the scientific literature, and archiving everything they find.
It's a huge amount of data. Take spores, for example. Renzaglia's team is recording, among other information, their shape and color, their "ornamentation" (whorls and ridges), their internal structure and development, and what types of molecules, such as proteins and sugars, are stored inside.
Using a scanning electron microscope, which bounces electrons off the surface of a sample, they're looking at plant structures such as stomata (pores) and sperm cells enlarged some 4,000 to 7,000 times-big enough to study the whip-like tails called flagella that enable the sperm to swim to their destination. Using a transmission electron microscope, which shoots electrons through a thin section of tissue, they're viewing the inner workings of cell nuclei and chloroplasts (the cell bodies that carry out photosynthesis). This work is being done under the aegis of SIUC's IMAGE facility.
Two tiny bryophyte species whose microanatomy differs considerably can look virtually identical to the naked eye. Conversely, one species can vary considerably in its outward appearance depending on environmental conditions. So cellular features will be key to learning how these early plants evolved.
For example, some hornworts have big chloroplasts that look identical to those in green algae, Renzaglia and Duff have found, while other hornworts have smaller chloroplasts but more of them. The latter species are farther away, evolutionarily, from the algae/hornwort split.
If this work sounds esoteric, consider that most advances in knowledge start out that way-and that life forms have surprising connections.
As an undergraduate in Renzaglia's lab, Kelly Wood studied plant sperm exposed to a mutation-causing chemical that damaged their flagella. As a result, these cells had trouble swimming. Fast forward: Wood went on to medical school, where she learned about human reproductive and respiratory cells with damaged cilia (hairs that move the cells themselves or surrounding material). She discovered that these cilia had the same kinds of damage as the plant flagella, which are structurally similar. Medical researchers now have a new experimental model that may shed light on the nature of the human disorder.
Intriguing information also will come from genetic studies of early land plants. Other team members are analyzing DNA from the same plant specimens that Renzaglia and O'Kelly are working with. Among other things, they are creating "reference libraries" of thousands of chromosome snippets from each specimen for cross-species comparison. The more genes that two organisms have in common, the more likely it is that they fall on the same branch or twig of the evolutionary tree. "If we understand these gene sequences [from early plants], it will help tell us how genomes change over time," Renzaglia says.
The genetic analyses will generate gigabyte after gigabyte of valuable data to be combined with the morphological data. Both are needed.
"Genes don't tell you what the plant looks like or how it functions," Renzaglia says. "And without information on morphology, you won't know what structural changes have taken place during evolution." Gene studies will help morphologists focus their efforts, and vice-versa. Although scientists ultimately will tease out the function of many genes, particularly those that are shared by many species, such analysis is time-consuming, and it can't be done for every gene in every kind of organism.
The fact that many species and even entire lineages have gone extinct over millions of years also complicates genetic analysis. "It's possible that all of the early hornworts died off except for one branch, which persisted unchanged for a long time and then underwent recent speciation," Renzaglia speculates by way of example. "Then the group would be old, but much of the genetic diversity would be recent. In colonizing land, probably thousands of successful species died off. It's frustrating-we only have bits and pieces [of evidence]."
Why not just study fossil plants? "You can't get DNA out of fossils," Renzaglia notes. "You can't see development; you can't see sperm. You can't, in many cases, see cellular details. And we don't have a good fossil record of the bryophytes-they're small and fragile."
Pinning down the details of plant evolution will take a grand synthesis of fossil, genetic, and morphological evidence. And that poses the team's biggest technical challenge: how to wade through all that data. The team will develop new methods of organizing, combining, and analyzing diverse data sets to draw conclusions about evolutionary history. Their Holy Grail, and a major goal of the NSF initiative, is an analytical model that can be applied to determine the evolutionary relationships of other organisms.
Besides Renzaglia and O'Kelly, scientists at the University of California - Berkeley, the University of Washington, Yale University, Utah State University, and Lawrence Berkeley National Laboratory are working on the project.
Renzaglia is passionate about this work. "It has the potential to uncover all kinds of new information," she says. "There's biodiversity at every level of life, including the cellular level. This research has opened up a world nobody's known about.
"We don't know what we're going to discover, but we know it will rewrite the textbooks."
Technorati : LIFE:, LUSH
NASA officials and launch managers were pleased Tuesday following a clean countdown and flawless launch of space shuttle Discovery from NASA's Kennedy Space Center in Florida.















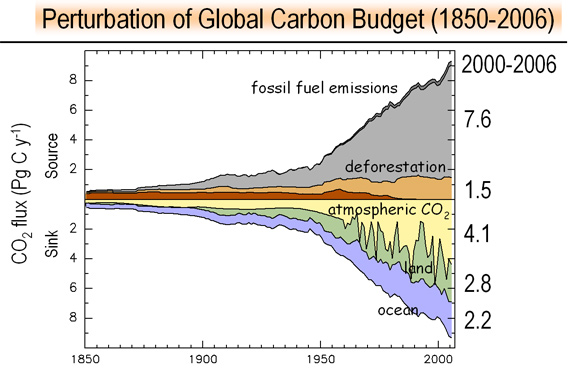 Carbon dioxide - the greenhouse gas considered most responsible for global warming - has been emitted into the Earth's atmosphere at a dramatically accelerating pace since 2000, researchers reported Monday.
Carbon dioxide - the greenhouse gas considered most responsible for global warming - has been emitted into the Earth's atmosphere at a dramatically accelerating pace since 2000, researchers reported Monday. Anthropogenic carbon emissions: World carbon intensity of GDP. Courtesy of the Global Carbon Project
Anthropogenic carbon emissions: World carbon intensity of GDP. Courtesy of the Global Carbon Project



